Therapeutic Effects of Ginseng and Doxorubicin on DMBA-Induced Ovarian Cancer in Wistar Rats
Main Article Content
Abstract
ABSTRACT
Background
Ovarian cancer can be attributed to various external factors, such as tobacco use, exposure to chemicals and radiation, and infections. Internal factors, including inherited mutations, hormonal factors, immune conditions, and random mutations, also play a role in its development. Exposure to carcinogen such as 7,12 Dimethyl Benz Anthracene can induce ovarian cancer. The aim of the study is to investigate the effect of Ginseng and Doxorubicin on Ovotoxicity induced by DMBA in Wistar rat. s
Methods
A total number of twenty-five females (25) wistar rats which average weight was 135g were used and the animals were randomly split into five groups (A, B, C, D, E) with each group comprising of five rats. Group A (served as the control) given physiological saline only, Group B were induced with 7,12 Dimethyl Benz Anthracene and post treated with Ginseng (50 mg/kg) (GIN). Group C were induced with 7,12 DMBA and post treated with Doxorubicin (25mg/kg). Group D were induced with 7,12 DMBA and post treated with Doxorubicin (25 mg/kg) and Ginseng (50 mg/kg). Group E were induced with 7,12
Dimethyl Benz (a) Anthracene (DMBA) (50mg/kg). Drug administration was done orally using oral cannula and intramuscularly using syringe and needle.
Results
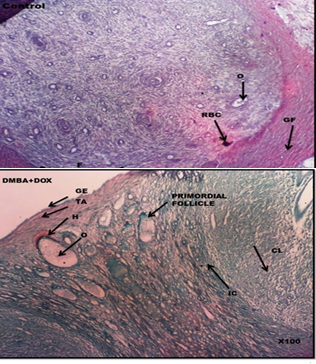
Ovary histoarchitecture for control group A, B (GIN group), and D (DOX+GIN group) revealed intact organization and structure of the ovary. In these groups, no observable pathological changes were seen as the ovaries were characterized with the presence of numerous follicular cells with a normal and well-defined cellular density. For C (DOX group), the histoarchitecture showed a localized collection of blood outside the blood vessels, typically resulting from a ruptured blood vessel (hematoma of blood vessels) and slight degenerative changes in the ovary. For E (DMBA group), showed atrophied and atretic ovarian cells, necrotic oocyte, severe degenerative changes were also observed.
For Hormonal analysis, there was a significant decrease in Serum FSH & LH concentration in DMBA treated group compared to the Ginseng and control groups.
Conclusions
DMBA evidently induced ovarian toxicity, and its effects were efficiently attenuated by the use of Ginseng and Doxorubicin.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
How to Cite
References
Anand P, Kunnumakara AB, Sundaram C, Harikumar KB, Tharakan ST, Lai OS, et al. Cancer is a preventable disease that requires major lifestyle changes. Pharm Res. 2008;25(9):2097-116. DOI: https://doi.org/10.1007/s11095-008-9661-9
Ferlay J, Shin HR, Bray F, Mathers 2010. Estimates of worldwide burden of cancer. Int J Cancer. 2013;127:2893-917. DOI: https://doi.org/10.1002/ijc.25516
Jedy-Agba E, Curado MP, Ogunbiyi O, Oga E, Fabowale T, Igbinoba F, et al. Cancer incidence in Nigeria: a report from population-based cancer registries. Cancer Epidemiol. 2012;36(5) DOI: https://doi.org/10.1016/j.canep.2012.04.007
Boggiti M, Penchalaneni J. Effect of Cinnamaldehyde on Female Reproductive Hormones in 7, 12 Dimethyl Benzanthracene Induced Ovarian Cancer Rats. 1989.
Miyata M, Kudo G, Lee YH, Yang TJ. Microsomal epoxide hydrolase is required for the carcinogenic activity of 7,12 DMBA. J Biol Chem. 1999;274:23963-8. DOI: https://doi.org/10.1074/jbc.274.34.23963
Gelboin HR, Fernandez. Targeted disruption of the microsomal epoxide hydrolase gene. J Biol Chem. 1980.
Gandlur SM, Thangavelu P, Srivastava N, Holmes N, Bhushan A. Membrane topology of the DrrB protein of the doxorubicin transporter of Streptomyces peucetius. J Biol Chem. 2004;279(26):27799-806. DOI: https://doi.org/10.1074/jbc.M402898200
Carvahlo RDS, Moura LDD, Geronimo G, Mendonça TC, Lima FFD, de Paula E. Antineoplastics encapsulated in nanostructured lipid carriers. Molecules. 2021;26(22):6929. DOI: https://doi.org/10.3390/molecules26226929
Blumenthal M. Herb sales down in mainstream market, up in natural food stores. HerbalGram. 2002;55:60.
Seghinsara AM, Khalaj Z, Rezvani ME, Norouzian M, Salehnia M. Panax ginseng extract improves follicular development after mouse preantral follicle 3D culture. Cell J (Yakhteh). 2019;21(2):210.
Choi JH, Kim SN, Kang DW, Kim ND, Yoo H, Lee MK. Korean red ginseng alleviates dehydroepiandrosterone-induced polycystic ovarian syndrome in rats via its anti-inflammatory and antioxidant activities. J Ginseng Res. 2020;44(6):790-8. DOI: https://doi.org/10.1016/j.jgr.2019.08.007
Adeleke O, Falana B, Babawale G, Atere T, Abayomi T, Olorunfemi T. Evaluation of the comparative effects of antihypertensive drugs: Methyldopa and Moringa oleifera leaves on the hypothalamic-pituitary-gonadal axis in male Wistar rats. J Exp Clin Anat. 2017;16(1):71-6.
Pink JJ, Planchon SM, Tagliarino C, Varnes ME, Siegel D, Boothman DA. NAD(P)Hoxidoreductase activity is the principal determinant of β-lapachone cytotoxicity. J Biol Chem. 2000;275:5416-24. doi: 10.1074/jbc.275.8.5416. DOI: https://doi.org/10.1074/jbc.275.8.5416
Burchiel SW, Davis DA, Ray SD, Barton SL. DMBA induces programmed cell death (apoptosis) in the A20.1 murine B cell lymphoma. Fundam Appl Toxicol. 1993;21:120-4. DOI: https://doi.org/10.1093/toxsci/21.1.120
Ward EC, Murray MJ, Lauer LD, House RV, Dean JH. Persistent suppression of humoral and cell-mediated immunity in mice following exposure to the polycyclic aromatic hydrocarbon 7, 12-dimethylbenz[a]anthracene. Int J Immunopharmacol. 1985;8(1):13-22. DOI: https://doi.org/10.1016/0192-0561(86)90068-8
World Health Organization. Global Cancer Observatory: Cancer Today. Lyon: International Agency for Research on Cancer; 2020.
Sobinoff AP, Mahony M, Nixon B, Roman SD, McLaughlin EA. Understanding the villain: DMBA-induced preantral ovotoxicity involves selective follicular destruction and primordial follicle activation through PI3K/Akt and mTOR signaling. Toxicol Sci. 2011 Oct;123(2):531-40. DOI: https://doi.org/10.1093/toxsci/kfr195
Razina CD, Miriyala S, Cole MP, Begley U, Butterfield DA, Vore M. Chemo Brain (Chemo Fog) as a Potential Side Effect of Doxorubicin Administration. Arch Toxicol. 2010;84(5):379-87.
Saha S, Chant D, Welham J, McGrath J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005;2(5). DOI: https://doi.org/10.1371/journal.pmed.0020141