Impact of Red Acalypha (Acalypha Wilkesiana) Extract on Weight, Oxidative Stress, Memory, and Hippocampal Structure in Sprague-Dawley Rats
Main Article Content
Abstract
Background
The therapeutic potential of medicinal plants in neurological health has gained attention. Acalypha wilkesiana, or Red Acalypha, has traditionally been used for anti-inflammatory, antimicrobial, and wound-healing properties. The hippocampus, a key brain region for memory, learning, and spatial navigation, is vulnerable to damage, making it a focus for studying natural compounds' effects on cognitive health. Damage to the hippocampus can impair cognitive abilities, emphasizing the need for interventions to prevent neurodegeneration.
Method
This study assessed Acalypha's effects on hippocampal health in rats. Four groups of seven rats each were used. The control group received distilled water, while the other groups were given Acalypha extracts at doses of 100 mg/kg, 200 mg/kg, or 400 mg/kg orally for 28 days after a 14-day acclimatization. The study measured body weight, spatial memory using the Morris water maze, oxidative stress markers (superoxide dismutase [SOD], glutathione [GSH], catalase [CAT], malondialdehyde [MDA]), and hippocampal histology.
Results
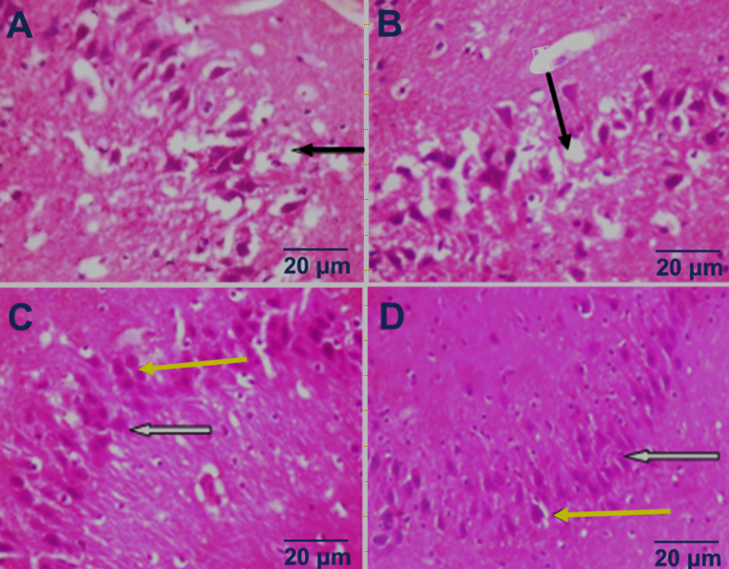
Relative to the control, Acalypha treatment significantly (p < 0.05) improved multiple parameters, including mitigating weight loss, enhancing spatial memory performance in the Morris water maze test, and reducing oxidative stress markers such as SOD, GSH, CAT, and MDA in a dose-dependent manner. Additionally, it provided neuroprotection to the hippocampus, as evidenced by a reduction in vacuolation compared to the control group.
Conclusions
Acalypha demonstrated notable neuroprotective effects, improving memory and reducing oxidative stress. This suggests its potential as a therapeutic agent for preserving hippocampal health and enhancing cognitive function, particularly against neurodegenerative conditions.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
How to Cite
References
Eze EF, Uzor PF, Obi BC, Osadebe PO. Nigerian medicinal plants with analgesic and anti-inflammatory potentials. African Journal of Pharmaceutical Research and Development. 2019;11:165-88.
Kumar S, Pandey AK. Chemistry and biological activities of flavonoids: an overview. The scientific world journal. 2013;1:162750.
Sivanesan I, Muthu M, Kannan A, Pushparaj SS, Oh JW, Gopal J. Identification of epigallocatechin-3-gallate (EGCG) from green tea using mass spectrometry. Separations. 2022 Aug 9;9(8):209.
Hambali A, Kumar J, Hashim NF, Maniam S, Mehat MZ, Cheema MS, Mustapha M, Adenan MI, Stanslas J, Hamid HA. Hypoxia-induced neuroinflammation in Alzheimer’s Disease: potential neuroprotective effects of Centella asiatica. Frontiers in Physiology, 2021 Oct 14;12:712317.
Rolls ET, Wirth S. Spatial representations in the primate hippocampus, and their functions in memory and navigation. Progress in neurobiology, 2018 Dec 1;171:90-113.
Siddiqui N, Sharma A, Kesharwani A, Parihar VK. Exploring role of natural compounds in molecular alterations Associated with Brain Ageing: a perspective towards Nutrition for Ageing Brain. Ageing Research Reviews. 2024 Mar 27:102282..
Rao YL, Ganaraja B, Murlimanju BV, Joy T, Krishnamurthy A, Agrawal A. Hippocampus and its involvement in Alzheimer’s disease: a review. 3 Biotech. 2022 Feb;12(2):55.
Cui XU, Zuo P, Zhang Q, Li X, Hu Y, Long J, Packer L, Liu J. Chronic systemic D‐galactose exposure induces memory loss, neurodegeneration, and oxidative damage in mice: Protective effects of R‐α‐lipoic acid. Journal of neuroscience research. 2006 Jun;83(8):1584-90.
Shoaib S, Ansari MA, Fatease AA, Safhi AY, Hani U, Jahan R, Alomary MN, Ansari MN, Ahmed N, Wahab S, Ahmad W. Plant-derived bioactive compounds in the management of neurodegenerative disorders: Challenges, future directions and molecular mechanisms involved in neuroprotection. Pharmaceutics. 2023 Feb 23;15(3):749.
Blaylock RL, Maroon J. Natural plant products and extracts that reduce immunoexcitotoxicity-associated neurodegeneration and promote repair within the central nervous system. Surgical Neurology International. 2012;3.
Olufunmilayo EO, Gerke-Duncan MB, Holsinger RD. Oxidative stress and antioxidants in neurodegenerative disorders. Antioxidants. 2023 Feb 18;12(2):517.
Akinloye DI, Sojinu OS, Ugbaja RN, Agemo S, Akintubuwa MO, Bolaji TJ. Appraisal of Acalypha wilkesiana Godseffiana mitigating effects against carbon tetrachloride (CCl 4)-induced oxidative impairment in female wistar rat. Advances in Traditional Medicine. 2021:1-20.
Howes MJ, Perry NS, Vásquez‐Londoño C, Perry EK. Role of phytochemicals as nutraceuticals for cognitive functions affected in ageing. British journal of pharmacology. 2020 Mar;177(6):1294-315.
Ibrahim M, Sadiq IZ, Abdu AM, Raphleen NC. Toxicity studies and effect of aqueous leaf extracts of Acalypha wilkesiana on the renal function in male albino rats. Journal of Experimental Research. 2020 Dec;8(4).
Ikewuchi JC. Moderation of hematological and plasma biochemical indices of sub-chronic salt-loaded rats, by an aqueous extract of the leaves of Acalypha wilkesiana ‘Godseffiana’Muell Arg (Euphorbiaceae). Asian Pacific journal of tropical medicine. 2013 Jan 1;6(1):37-42.
Morris R. Developments of a water-maze procedure for studying spatial learning in the rat. Journal of neuroscience methods. 1984 May 1;11(1):47-60.
Sun M, Zigman S. An improved spectrophotometric assay for superoxide dismutase based on epinephrine autoxidation. Analytical biochemistry. 1978 Oct 1;90(1):81-9.
Sedlak J, Lindsay RH. Estimation of total, protein-bound, and nonprotein sulfhydryl groups in tissue with Ellman's reagent. Analytical biochemistry. 1968 Jan 1;25:192-205.
Aebi H. [13] Catalase in vitro. InMethods in enzymology 1984 Jan 1 (Vol. 105, pp. 121-126). Academic press.
Samarghandian S, Azimi-Nezhad M, Farkhondeh T, Samini F. Anti-oxidative effects of curcumin on immobilization-induced oxidative stress in rat brain, liver and kidney. Biomedicine & Pharmacotherapy. 2017 Mar 1;87:223-229.
Idowu OK, Ajayi SO, Oluyomi OO, Atobatele NM, Okesina AA. Effect of Fenofibrate on Hippocampal Insulin Resistance and Cognitive Function in a Rat Model of Type 2 Diabetes Mellitus. Journal of Krishna Institute of Medical Sciences (JKIMSU). 2021 Jan 1;10(1).
Idowu OK, Oluyomi OO, Faniyan OO, Dosumu OO, Akinola OB. The synergistic ameliorative activity of peroxisome proliferator‐activated receptor‐alpha and gamma agonists, fenofibrate and pioglitazone, on hippocampal neurodegeneration in a rat model of insulin resistance. Ibrain. 2022 Sep;8(3):251-63..
Tontonoz P, Spiegelman BM. Fat and beyond: the diverse biology of PPARγ. Annu. Rev. Biochem.. 2008 Jul 7;77(1):289-312.
Marrelli M, Conforti F, Araniti F, Statti GA. Effects of saponins on lipid metabolism: A review of potential health benefits in the treatment of obesity. Molecules. 2016 Oct 20;21(10):1404.
Matthews B.R. Memory dysfunction. CONTINUUM: Lifelong Learning in Neurology, 2015; 21(3):613-626
Bliss TV, Collingridge GL. Expression of NMDA receptor-dependent LTP in the hippocampus: bridging the divide. Molecular brain. 2013 Dec;6:1-4.
Wang S, Bian L, Yin Y, Guo J. Targeting NMDA receptors in emotional disorders: their role in neuroprotection. Brain Sciences. 2022 Sep 30;12(10):1329.
Wojtunik-Kulesza K, Oniszczuk T, Mołdoch J, Kowalska I, Szponar J, Oniszczuk A. Selected natural products in neuroprotective strategies for Alzheimer’s disease—a non-systematic review. International Journal of Molecular Sciences. 2022 Jan 21;23(3):1212.
Jové M, Mota-Martorell N, Pradas I, Martín-Gari M, Ayala V, Pamplona R. The advanced lipoxidation end-product malondialdehyde-lysine in aging and longevity. Antioxidants. 2020 Nov 15;9(11):1132.
Jomova K, Alomar SY, Alwasel SH, Nepovimova E, Kuca K, Valko M. Several lines of antioxidant defense against oxidative stress: antioxidant enzymes, nanomaterials with multiple enzyme-mimicking activities, and low-molecular-weight antioxidants. Archives of Toxicology. 2024 May;98(5):1323-67.
Familoni OB, Asekun OT, Okoh O, Asekunowo AK, Ashafa AO. Polyphenolic constituents, antioxidant and hypoglycaemic potential of leaf extracts of Acalypha godseffiana from Eastern Nigeria: In vitro study. Journal of Medicinal Plants for Economic Development. 2019 Jan 1;3(1):1-9.
Didunyemi MO, Adetuyi BO, Oyewale IA. Inhibition of lipid peroxidation and in-vitro antioxidant capacity of aqueous, acetone and methanol leaf extracts of green and red Acalypha wilkesiana Muell Arg. Int J Biol Med Res. 2020;11(3):7089-94.
Vauzour D, Vafeiadou K, Rodriguez-Mateos A, Rendeiro C, Spencer JP. The neuroprotective potential of flavonoids: a multiplicity of effects. Genes & nutrition. 2008 Dec;3:115-26.
Sirisha S, Nikitha K. Neuropsychological screening on Acalypha indica whole plant extract. Journal of Young Pharmacists. 2020;12(2s):s82.
Kingsley O, Marshall AA, Inegbenose II. Phytochemical, proximate and elemental analysis of Acalypha wilkesiana leaves. Scientific Journal of Pure and Applied Sciences. 2013;2(9):323-31.