Herbicide residues as a possible risk factor in semen quality and spermatogenesis
Main Article Content
Abstract
Background
Reports from various countries have consistently demonstrated a correlation between herbicide exposure, arising from agricultural practices, and a decline in semen quality, leading to male infertility. This study was conducted at Ekiti State University Teaching Hospital in Ado-Ekiti, a rural community characterized by a predominantly agrarian population to determine the relationship between semen quality and herbicides residues.
Methods
The study focused on males whose spouses were seeking assistance at infertility clinics. Routine semen analyses were performed according to the World Health Organization (WHO) criteria, categorizing samples into normospermic, asthenospermic, oligospermic, and azoospermic groups. Seminal plasma samples from each group (twenty samples per group) were subjected to analysis for the presence and concentration of herbicides using High-Performance Liquid Chromatography (HPLC). The following herbicides were investigated: halosulfurum, linuron, fluometuron, chlo-rimuron, imaxamox, cloransulam, dicamba, fluroxypor, trichlopyr, propanil, cloclinafop, clethodim, quizalofop, fluazifop, pinoxaden, bentazon, atrazine, and bromoxynil. The obtained results were subjected to statistical analysis using SPSS version 24.
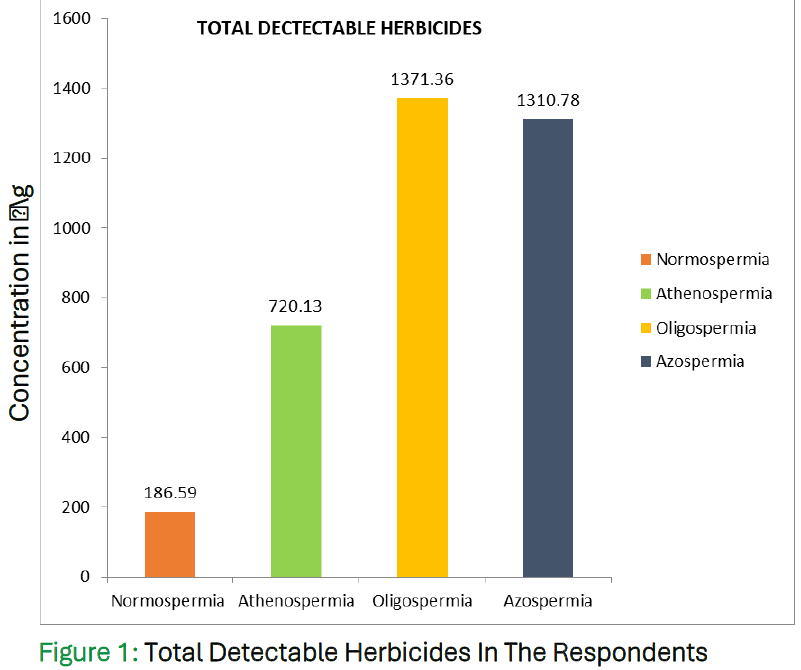
Results
The analysis revealed significantly higher concentrations of most herbicides in the asthenospermia, oligospermia, and azoospermia groups compared to the normospermic group (P<0.05). These findings suggest a strong association between herbicide exposure and poor semen quality in the studied population.
Conclusion
This study provides compelling evidence supporting the hypothesis that herbicides exposure could be a contributory factor to diminished semen quality in the investigated rural com-munity. The results underscore the importance of considering seminal herbicide determination as a routine component in male infertility testing. Additionally, the study advocates for the implementation of relevant legislation to mitigate potential risks associated with herbicide exposure.
Downloads
Article Details

This work is licensed under a Creative Commons Attribution 4.0 International License.
This work is licensed under a Creative Commons Attribution 4.0 International (CC BY 4.0) license. To view a copy of this license, visit https://creativecommons.org/licenses/by/4.0/.
How to Cite
References
References
De Jonge C, Barratt C L R. The present crisis in male reproductive health: an urgent need for a political, social, and research roadmap. Andrology 2019; 7(6), 762–768. DOI: https://doi.org/10.1111/andr.12673
Hossain F, Ali O, D'Souza U J, Naing D K. Effects of pesticide use on semen quality among farmers in rural areas of Sabah, Malaysia. Journal of occupational health. 2010; 52(6), 353–360. DOI: https://doi.org/10.1539/joh.L10006
Vander Borght M, Wyns C. Fertility and infertility: Definition and epidemiology. Clin Biochem. 2018;62:2-10. d DOI: https://doi.org/10.1016/j.clinbiochem.2018.03.012
Nateghian Z, Aliabadi E. Aspects of Environmental Pollutants on Male Fertility and Sperm Parameters. J Environ Treat Tech 2020;8(1):299–309.
Sciorio R, Tramontano L, Adel M, Fleming S. Decrease in Sperm Parameters in the 21st Century: Obesity, Lifestyle, or Environmental Factors? An Updated Narrative Review. Journal of personalized medicine 2024; 14(2), 198. DOI: https://doi.org/10.3390/jpm14020198
Stehle S, Bline A, Bub S, Petschick LL, Wolfram J, Schulz R, et al. Aquatic pesticide exposure in the U.S. as a result of non-agricultural uses. Environ Int. Dec 2019;133(Pt B):105234. DOI: https://doi.org/10.1016/j.envint.2019.105234
Ross, MA, Childs DJ. Herbicide Mode-of-Action Summary. Purdue University, Department of Botany: Plant Pathology, West Lafayette IN. Report No. WS-23-W. 1996.
de Araújo-Ramos AT, Passoni MT, Romano MA, Romano, RM, Martino-Andrade AJ, et al. Controversies on Endocrine and Reproductive Effects of Glyphosate and Glyphosate-Based Herbicides: A Mini-Review. Front Endocrinol (Lausanne). 2021;12:627210. DOI: https://doi.org/10.3389/fendo.2021.627210
Zhu L, Li W, Zha J, Wang, Z, et al. Dicamba affects sex steroid hormone level and mRNA expression of related genes in adult rare minnow (Gobiocypris rarus) at environmentally relevant concentrations. Environ Toxicol. 2015;30(6):693-703. DOI: https://doi.org/10.1002/tox.21947
Trentacoste SV, Friedmann AS, Youker RT, Breckenridge CB, Zirkin BR, et al. Atrazine effects on testosterone levels and androgen-dependent reproductive organs in peripubertal male rats. J Androl. 2001;22(1):142-8. DOI: https://doi.org/10.1002/j.1939-4640.2001.tb02164.x
Sengupta P, Banerjee R. Environmental toxins: Alarming impacts of pesticides on male fertility.Human & Experimental Toxicology. 2014;33(10):1017-1039. DOI: https://doi.org/10.1177/0960327113515504
Kojima H, Katsura E, Takeuchi S, Niiyama K, Kobayashi, K, et al. Screening for estrogen and androgen receptor activities in 200 pesticides by in vitro reporter gene assays using Chinese hamster ovary cells. Environ. Health Perspect. 2004;112, 524–531. DOI: https://doi.org/10.1289/ehp.6649
Prathima P, Venkaiah K, Daveedu T, Pavani R, Sukeerthi S, Gopinath M, et al. α-lipoic acid protects testis and epididymis against linuron-induced oxidative toxicity in adult rats. Toxicol Res. 2020;36(4):343-357. DOI: https://doi.org/10.1007/s43188-019-00036-y
Kim M, An G, Lim W, Song G, et al., Fluroxypyr-1-methylheptyl ester induced ROS production and mitochondrial apoptosis through the MAPK signaling cascade in porcine trophectoderm and uterine luminal epithelial cells. Pestic Biochem Physiol. 2022;187:105196 DOI: https://doi.org/10.1016/j.pestbp.2022.105196
Ore A, Olayinka ET. Fluazifop- p-butyl, an aryloxyphenoxypropionate herbicide, diminishes renal and hepatic functions and triggers testicular oxidative stress in orally exposed rats. Toxicol Ind Health. 2016;33(5):406-415. DOI: https://doi.org/10.1177/0748233716657763
El-Nagar MF, Elsisi AE. Exposure to bromoxynil octanoate herbicide induces oxidative stress, inflammation, and apoptosis in testicular tissue via modulating NF-кB pathway. Food Chem Toxicol. 2023 DOI: https://doi.org/10.1016/j.fct.2023.114008
Taşkıran M. Is There an Association Between Dietary Antioxidant Levels and Sperm Parameters in Male Infertility?. Cureus 2023; 15(8), e44339. https://doi.org/10.7759/cureus.44339 DOI: https://doi.org/10.7759/cureus.44339
Chyra-Jach D, Kaletka Z, Dobrakowski M, Machoń-Grecka A, Kasperczyk S, Birkner E, Kasperczyk A. The Associations between Infertility and Antioxidants, Proinflammatory Cytokines, and Chemokines. Oxidative medicine and cellular longevity; 2018, 8354747. DOI: https://doi.org/10.1155/2018/8354747
Zhu H, Wang H, Cheng Y, Liu D, Zhang A, Wen Z, Gao,J. Hadh deficiency induced oligoasthenoteratozoospermia through the TNF-α/Bcl-2 pathway in male mice. FASEB journal : official publication of the Federation of American Societies for Experimental Biology 2022: 36(12). DOI: https://doi.org/10.1096/fj.202201144R
Ventimiglia E, Viganò P, Alfano M, Abbate C, Cornelius J, Mattei A, Montorsi F, Salonia A. Correlation among isolated teratozoospermia, sperm DNA fragmentation and markers of systemic inflammation in primary infertile men. PloS one, 2021;16(6), e0251608. https://doi.org/10.1371/journal.pone.0251608 DOI: https://doi.org/10.1371/journal.pone.0251608
McIntyre BS, Barlow NJ, Foster PM, et al. Male rats exposed to linuron in utero exhibit permanent changes in anogenital distance, nipple retention, and epididymal malformations that result in subsequent testicular atrophy. Toxicol Sci. 2002;65(1):62-70. DOI: https://doi.org/10.1093/toxsci/65.1.62
Dcunha R, Kumari S, Najar MA, Aravind A, Suvarna KS, Hanumappa A, et al. High doses of clethodim-based herbicide GrassOut Max poses reproductive hazard by affecting male reproductive function and early embryogenesis in Swiss albino mice. 2023;336:139215. DOI: https://doi.org/10.1016/j.chemosphere.2023.139215
EFSA Scientific Committee. Scientific Opinion on the hazard assessment of endocrine disruptors: scientific criteria for identification of endocrine disruptors and appropriateness of existing test methods for assessing effects mediated by these substances on human health and the environment. 2013;11(3):3132. DOI: https://doi.org/10.2903/j.efsa.2013.3132
European Food Safety Authority (EFSA); Arena M, Auteri D, Barmaz S, Brancato A, Brocca D, Bura L, et al. Peer review of the pesticide risk assessment of the active substance clodinafop (variant evaluated clodinafop-propargyl). 2018;16(11):e05467. DOI: https://doi.org/10.2903/j.efsa.2018.5467
Shen Y, Zhang J, Xie J, Liu J, et al. In vitro assessment of corticosteroid effects of eight chiral herbicides. J Environ Sci Health 2019;55(2):91-102. DOI: https://doi.org/10.1080/03601234.2019.1665408
Zhang Y, Zhang J, Shi B, Li B, Du Z, Wang J, et al. Effects of cloransulam-methyl and diclosulam on soil nitrogen and carbon cycle-related microorganisms. Journal of Hazardous Materials 2021;418 DOI: https://doi.org/10.1016/j.jhazmat.2021.126395
Meftaul IM, Venkateswarlu K, Dharmarajan R, Annamalai P, Asaduzzaman M, Parven A, et al. Controversies over human health and ecological impacts of glyphosate: Is it to be banned in modern agriculture? Environ Pollution. 2020;263(Pt A):114372. DOI: https://doi.org/10.1016/j.envpol.2020.114372
Sharpe RM. Environmental/lifestyle effects on spermatogenesis. Philos Trans R Soc Lond B Biol Sci; 2010;365(1546):1697–1712. DOI: https://doi.org/10.1098/rstb.2009.0206
Kolawole OD, Okorie VO, Ogidiowa MT, Adeogun MO. Ethno-veterinary practices amongst small-holder farmers in Ekiti state, Nigeria. Afr J Tradit Complement Altern Med; 2007 10;4(4):434-42. DOI: https://doi.org/10.4314/ajtcam.v4i4.31238
Olayanju AO, Chris NI, Emmanuel AE, Oyetunde AB, Adebisi KY, Okolo CS. Prevalence of alloantibodies associated with haemolytic disease of the fetus and newborn in pregnant women at the Ekiti State University Teaching Hospital, Ado-Ekiti, Southwest Nigeria. Afr J Reprod Health. 2023 27(6s):70-78.
World Health Organisation. WHO laboratory manual for the examination and processing of human semen, Sixth Edition 2021. ISBN 978-92-4-003078-7 (electronic version).
Johnson CL, Jazan E, Kong SW, Pennell KD, et al. A two-step gas chromatography-tandem mass spectrometry method for measurement of multiple environmental pollutants in human plasma. Environ Sci Pollut Res Int 2020;28(3):3266-3279. DOI: https://doi.org/10.1007/s11356-020-10702-6
IBM Corp. IBM SPSS Statistics for Windows, Version 24.0. Armonk, NY: IBM Corp; 2016
Wathes DC, Abayasekara DR, Aitken DJ, et al. Polyunsaturated fatty acids in male and female reproduction. Biology of reproduction, 2007;77(2):190-201. DOI: https://doi.org/10.1095/biolreprod.107.060558
Storey BT. Biochemistry of the induction and prevention of the lipoperoxidative damage in human spermatozoa. Mol. Tium Reprod 1997;3:203-214. DOI: https://doi.org/10.1093/molehr/3.3.203
Kumar, N. “Sperm Mitochondria, the Driving Force Behind Human Spermatozoa Activities: Its Functions and Dysfunctions - A Narrative Review.” Current molecular medicine vol. 23,4 2023: 332-340. DOI: https://doi.org/10.2174/1566524022666220408104047
Hayes T B, Collins A, Lee M, Mendoza M, Noriega N, Stuart AA, Vonk A. Hermaphroditic, demasculinized frogs after exposure to the herbicide atrazine at low ecologically relevant doses. Proceedings of the National Academy of Sciences of the United States of America vol. 2002;99 (8) 5476-80. DOI: https://doi.org/10.1073/pnas.082121499
Ikeji CN, Adedara IA, Farombi EO. Dietary myricetin assuages atrazine-mediated hypothalamic-pituitary-testicular axis dysfunction in rats. Environ Sci Pollut Res Int. 2023;30(6):15655-15670. DOI: https://doi.org/10.1007/s11356-022-23033-5
de Liz Oliveira Cavalli VL, Cattani D, Heinz Rieg CE, Pierozan P, Zanatta L, Benedetti Parisotto E, Wilhelm Filho D, Mena Barreto Silva FR, Pessoa-Pureur R, Zamoner A. Roundup disrupts male reproductive functions by triggering calcium-mediated cell death in rat testis and Sertoli cells. Free Radical Biology and Medicine;2013; 65:335-346 DOI: https://doi.org/10.1016/j.freeradbiomed.2013.06.043
Klinefelter GR, Strader LF, Suarez JD, Roberts NL. Changes in calcium levels in rat testicular cells after atrazine treatment. Reprod Toxicol. 2006;22(4):584-591
Guidobaldi HA, Teves ME, Uñates DR, Anastasía A, Giojalas LC. Progesterone from the cumulus cells is the sperm chemoattractant secreted by the rabbit oocyte cumulus complex. PLoS One. 2017;12(5):e0171693
Choy J T, Amory J K. Nonsurgical Management of Oligozoospermia. The Journal of clinical endocrinology and metabolism 2020;1;105(12):e4194–207 DOI: https://doi.org/10.1210/clinem/dgaa390
Colpi G M, Francavilla S, Haidl G, Link K, Behre H M, Goulis D G, Krausz C, Giwercman A. European Academy of Andrology guideline Management of oligo-astheno-teratozoospermia. Andrology 2018; 6(4), 513–524. DOI: https://doi.org/10.1111/andr.12502
Liu H, Zheng H, Li Y, Tang Y, Peng H, Li Q, Zhuang J, Zhou Y, Zhou, Y, Tu X, Zhang X. Seminal testosterone, rising viewpoint of local spermatogenesis in nonobstructive azoospermia: One center long-term bidirectional cohort study. Frontiers in endocrinology 2022) 13, 992556. DOI: https://doi.org/10.3389/fendo.2022.992556
Swan SH. Semen quality in fertile US men in relation to geographical area and pesticide exposure. Int J Androl 2006; 29: 62–68. DOI: https://doi.org/10.1111/j.1365-2605.2005.00620.x
Betancourt M, Resendiz A, Casas E and Fierro R(2006) “Effects of two insecticides and two herbicides on the porcine sperm motility patterns using computer associated semen analysis (CASA) in vitro “ Reproductive toxicology 22(3) 508-512. DOI: https://doi.org/10.1016/j.reprotox.2006.03.001
Hayes T B, Anderson L L, Beasley V R, de Solla S R, Iguchi T, Ingraham H et al. Demasculinization and feminization of male gonads by atrazine: consistent effects across vertebrate classes. The Journal of steroid biochemistry and molecular biology 2011; 127(1-2), 64–73. DOI: https://doi.org/10.1016/j.jsbmb.2011.03.015
Zhang J, Begum A, Brännström K, Grundström C, Iakovleva I, Olofsson A, Sauer-Eriksson AE, Andersson PL. Structure-Based Virtual Screening Protocol for in Silico Identification of Potential Thyroid Disrupting Chemicals Targeting Transthyretin. Environ Sci Technol. 2016 Nov 1;50(21):11984-11993. DOI: https://doi.org/10.1021/acs.est.6b02771
La Vignera S, Vita R. Thyroid dysfunction and semen quality. Int J Immunopathol Pharmacol. 2018 Jan-Dec;32:2058738418775241. DOI: https://doi.org/10.1177/2058738418775241
Salazar KD, Miller MR, Barnett JB, Schafer R. Evidence for a novel endocrine disruptor: the pesticide propanil requires the ovaries and steroid synthesis to enhance humoral immunity. Toxicol Sci. 2006; 93(1):62-74. DOI: https://doi.org/10.1093/toxsci/kfl038
Nowak K, Jabłońska E, Ratajczak-Wrona W. Immunomodulatory effects of synthetic endocrine disrupting chemicals on the development and functions of human immune cells. Environ Int. 2019 125:350-364. DOI: https://doi.org/10.1016/j.envint.2019.01.078
Kongtip P, Nankongnab N, Kallayanatham N, Pundee R, Choochouy N, Yimsabai J, Woskie S. Thyroid Hormones in Conventional and Organic Farmers in Thailand. Int J Environ Res Public Health. 2019; 29;16(15):2704. DOI: https://doi.org/10.3390/ijerph16152704
Cocuzza, M., Alvarenga, C., & Pagani, R. (2013). The epidemiology and etiology of azoospermia. Clinics (Sao Paulo, Brazil), 68 Suppl 1(Suppl 1), 15–26. https://doi.org/10.6061/clinics/2013(sup01)03 DOI: https://doi.org/10.6061/clinics/2013(Sup01)03
Komsky-Elbaz A, Kalo D, Roth Z. (2021). Carryover effect of atrazine and its metabolite-from treated bovine spermatozoa to the embryo's transcriptome†. Biology of reproduction; 104(5), 1162–1180. DOI: https://doi.org/10.1093/biolre/ioab027
Omran N E, Salama W M. The endocrine disruptor effect of the herbicides atrazine and glyphosate on Biomphalaria alexandrina snails. Toxicology and industrial health 2016; 32(4), 656–665. DOI: https://doi.org/10.1177/0748233713506959
Ahrens WH. Herbicide handbook. Seventh Edition 1994. [Google Scholar] [Ref list
Suvarchala G, and Philip GH. Toxicity of 3,5,6-trichloro-2-pyridinol tested at multiple stages of zebrafish (Danio rerio) development. Environ Sci Pollut Res Int. 2016 23(15):15515-23. DOI: https://doi.org/10.1007/s11356-016-6684-3
Sevim Ç, Çomaklı S, Taghizadehghalehjoughi A, Özkaraca M, Mesnage R, Kovatsi L, Burykina TI, Kalogeraki A, Antoniou MN, Tsatsakis A. An imazamox-based herbicide causes apoptotic changes in rat liver and pancreas. Toxicol Rep. 2018 ; 19;6:42-50. DOI: https://doi.org/10.1016/j.toxrep.2018.11.008
Jin J, Kurobe T, Ramírez-Duarte WF, Bolotaolo MB, Lam CH, Pandey PK, Hung TC, Stillway ME, Zweig L, Caudill J, Lin L, Teh SJ. Sub-lethal effects of herbicides penoxsulam, imazamox, fluridone and glyphosate on Delta Smelt (Hypomesus transpacificus). Aquat Toxicol. 2018 ;197:79-88. doi: 10.1016/j.aquatox.2018.01.019. Epub 2018 Feb 1. PMID: 29448126. DOI: https://doi.org/10.1016/j.aquatox.2018.01.019
Shen Y, Zhang J, Xie J, Liu J. In vitro assessment of corticosteroid effects of eight chiral herbicides. J Environ Sci Health B. 2020;55(2):91-102. DOI: https://doi.org/10.1080/03601234.2019.1665408
Tsatsakis A, Tyshko NV, Docea AO, Shestakova SI, Sidorova YS, Petrov NA, Zlatian O, Mach M, Hartung T, Tutelyan VA. The effect of chronic vitamin deficiency and long term very low dose exposure to 6 pesticides mixture on neurological outcomes - A real-life risk simulation approach. Toxicol Lett. 2019; 15;315:96-106. doi: 10.1016/j.toxlet.2019.07.026. Epub 2019 Aug 3. PMID: 31386889. DOI: https://doi.org/10.1016/j.toxlet.2019.07.026
Dai Z, Wu Z, Hang S, Zhu W, Wu G. Amino acid metabolism in intestinal bacteria and its potential implications for mammalian reproduction. Molecular human reproduction. 2015; 21(5), 389–409. DOI: https://doi.org/10.1093/molehr/gav003
Hou Y, Yao K, Yin Y, Wu G. Endogenous Synthesis of Amino Acids Limits Growth, Lactation, and Reproduction in Animals. Advances in nutrition (Bethesda, Md.) 2016 7(2), 331–342. DOI: https://doi.org/10.3945/an.115.010850
Storey B T. Biochemistry of the induction and prevention of the lipoperoxidative damage in human spermatozoa. Mol. Tium Reprod 1997; 3: 203-214. DOI: https://doi.org/10.1093/molehr/3.3.203
Kaundun, S S. Resistance to acetyl CoA carboxylase inhibiting herbicides. Pest Management Science 2014; 70(9), 1405-1417. DOI: https://doi.org/10.1002/ps.3790
Ahmadivand S, Farahmand H, Teimoori-Toolabi L, Mirvaghefi A, Eagderi S, Geerinckx T, Shokrpoor S, Rahmati-Holasoo H. Boule gene expression underpins the meiotic arrest in spermatogenesis in male rainbow trout (Oncorhynchus mykiss) exposed to DEHP and butachlor. General and comparative endocrinology,2016; 225, 235–241. DOI: https://doi.org/10.1016/j.ygcen.2015.05.011
Luetjens C M, Xu EY, Rejo Pera R A, Kamischke A, Nieschlag E, Gromoll J. Association of meiotic arrest with lack of BOULE protein expression in infertile men. The Journal of clinical endocrinology and metabolism. 2004; 89(4), 1926–1933. DOI: https://doi.org/10.1210/jc.2003-031178
Le J, Lei X, Ren Y, Li Z, Tu H, Ding F, Yi X, Zhou Y, Liu Q, Zhang S. Exogenous oestradiol benzoate induces male mice azoospermia through modulation of oxidative stress and testicular metabolic cooperation. Molecular medicine reports 2019; 19(6), 4955–4963. DOI: https://doi.org/10.3892/mmr.2019.10169