Dermatofibrosarcoma Protuberans of the Lid with Orbital Invasion: The Challenges of Late Presentation - Case Report
Yusuf Ibrahim Abiodun, Oladele-Abdulhameed Sofiyyahand Owoeye Joshua Foluso
Department of Ophthalmology, University of Ilorin Teaching Hospital, Ilorin, Nigeria.
Corresponding Author
Dr Yusuf I. A.
Department of Ophthalmology,
University of Ilorin Teaching Hospital, Ilorin, Nigeria
+234-8061242353
Running Title: Lid Dermatofibrosarcoma Protuberans: Late Presentation and Orbital Invasion
Abstract
Dermatofibrosarcoma Protuberans (DFSP) is a very rare painless intermediate to low grade malignancy of the subcutaneous tissue or dermis commonly seen in the young to middle age group and affecting the trunk and proximal extremities. The aim of this report is to present the rare lid affectation of the tumour and the challenges involved in managing a locally invasive tumour amidst late presentation and financial limitations.
This is a case report of a 35-year-old woman who presented with a 10-year history of Dermatofibrosarcoma Protuberans (DFSP) of the right lower lid with facial, maxillary, and orbital invasion of the right side. She underwent multiple surgeries, including orbital exenteration. There were limitations in accessing and following primary surgical procedures with radiation therapy and chemotherapy that could have impacted the overall outcome of this case.
Managing a DFSP in the presence of late presentation, marked local invasiveness and financial constraints is challenging. A multidisciplinary approach involving social workers must be employed if a cure is to be achieved.
Key words:Dermatofibrosarcoma protuberans, orbital invasion, late presentation, exenteration, lid dermatofibrosarcoma protuberans
INTRODUCTION
Dermatofibrosarcoma Protuberans (DFSP) (also known as Darier-Ferrand Tumour or Darier-Hoffmann Tumour) is a very rare painless intermediate to low-grade malignancy of subcutaneous tissue or dermis characterised by slow infiltrative growth with tumour growth spanning decades 1, little metastatic potential, but a high tendency to recur locally after surgical excision. 2 It was first described by Darier and Ferrand in 1924 who labelled it "progressive and recurring dermatofibroma”. 3 It is most commonly seen in the trunk followed by the proximal extremities and the head and neck. 4,5 It is commonly seen in young to middle-aged individuals, although it has been described in both children and elderly 6 with a slight male preponderance. 7 DFSP comprises roughly 0.01% of all malignant tumours and approximately 2 to 6 percent of all soft-tissue sarcomas. 8 The prevalence of DFSP is unknown in this environment and other developing nations. In the United states, the estimated incidence is 4.2 cases per million persons annually with varying rates between the white and black population . 9,10 The incidence among blacks (6.5 per million) is almost double that among whites (3.9 per million) population. 10 Even though no definitive risk factors have been reported, factors such as site of trauma, region of extensive burns, surgical incision sites, vaccinations sites (BCG vaccination), arsenic poisoning have all been considered associations and further studies to understand these associations are currently being explored. 7
Treatment involving Mohs micrographic surgery (MMS) or wide surgical excision is the standard. 7 Ratner et al while studying the 3-dimensional reconstruction of DFSP reported that the tumour can have irregular shapes and extend in a villous or finger-like manner 11 and this finger like extension of the tumour may be responsible for the local recurrence in these patients. 2
This is a case presentation of a woman with late presentation of lower lid DFSP who later developed orbital and maxillary infiltration.
CASE PRESENTATION
A 35-year-old trader presented to our facility with a 10-year history of slowly progressive and painless swelling of the right lower lid. There was a history of lid trauma sustained during a fight a month before the swelling. The swelling of the lid progressively increased in size, occluding not only her visual axis but also the entire right globe, significantly affecting her vision, causing her to come for care. There was no history of ulceration or bleeding from the lesion, no associated swelling in the left eye (from which she enjoys good vision) or any part of the body. There was no symptoms suggestive of intracranial or pulmonary involvement.
Examination revealed a right facial mass involving the lower lid, maxilla, and zygomatic area measuring 11 cm by 11 cm by 7cm, firm, non-tender with slightly hypopigmented overlying skin (Figure 1). There was no peripheral lymphadenopathy.
She was reviewed by colleagues in the Ear, Nose, and Throat (ENT) and maxillofacial units, had an orbito-cranial computed tomographic scan which revealed the extent of the tumour with no intra-cranial, sinus or orbital involvement and was planned for an initial incisional biopsy. Histology revealed cells with an elongated and moderate amount of eosinophilic cytoplasm, proliferating spindle-shaped disposed in a storiform pattern, interdigitated with fat lobules reminiscent of the honeycomb appearance, and collagenous tissue within the stroma suggestive of DFSP (figure 2). Due to the extensiveness of the mass and lack of MMS facility in the institution, she had debulking of the potato-like highly vascularized mass with reconstruction of the face and lid and was immediately commenced on Imatinib at a dose of 600mg/day and radiotherapy at a dosage of 50 Gray delivered over multiple fractions for total tumour clearance. Following the surgery (figure 3), she had a right eye vision of 6/60 with mild limitation of extra-ocular motility globally. However, she was unable to maintain the institutional line of management (chemotherapy and radiotherapy) due to financial constraints. Approximately 7 months after surgery, she developed regrowth of the mass with invasion of the right orbit, which warranted a repeat excision and exenteration of the orbit. However, she was lost to follow-up.
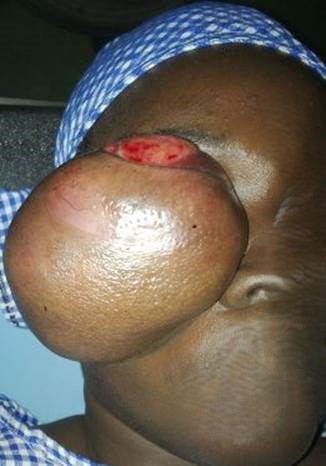
Figure 1: Large facial mass measuring 11cm×11cm×7cm
with exposed keratinized conjunctiva
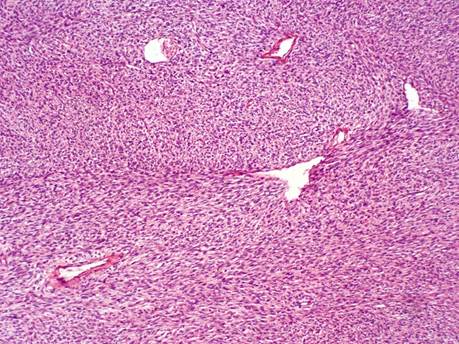
Figure 2: Histology of the tissue
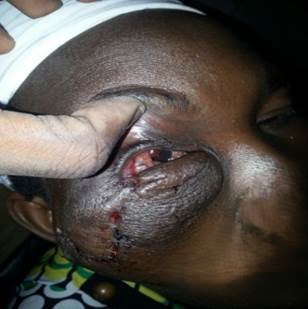
Figure 3: Showing the globe 3 days after first excision.
DISCUSSION
The management of DFSP is challenging when the patient is presenting with an advanced tumour and limited financial resources to effectively pursue treatment course. The slow growth and painless nature of the tumour, and ignorance has contributed to the late presentation of this case. Recurrence of the mass makes the treatment challenging and frustrating to both the managing team and the patients. The patient in question had undergone excision of the tumour twice and right orbital exenteration without total tumour clearance emphasizing the importance of adjuvant and other supportive measures in achieving a cure in advanced cases.
This is the first case of DFSP that will be managed in the Oculoplastic unit of our hospital. This emphasizes the rarity of the tumour in the ophthalmic domain. Monsudi et al in North-western Nigeria reported a case also involving the right lid and scalp as well as the challenges involved in the management. 12 Many studies across the country have documented a predilection for the trunk. A study by Effiom et al in Lagos, Nigeria revealed that of the 191 soft tissue sarcomas managed in 13 years, 14.7% of the cases were DFSP with trunk predilection and facial involvement in only 3 cases (1.6%). 13 Similarly, another study at the University College Hospital Ibadan revealed that over a period of 27 years, sixty-nine cases of DFSP were managed with a slight male and truncal predilection. 14
DFSP has a deceptively benign early clinical presentation and displays an indolent course for many years before exhibiting its true nature. Its local invasiveness results in early recurrence if prompt, wide excision is not performed. 15 Since it is a slow growing tumour, the duration of development ranges from weeks to years. Delay in diagnosis and clinical misdiagnosis of the initial lesion is common and is due to absence of symptoms as only 10-25% of these patients experience pain and tenderness. 16
The treatment options include surgery, radiotherapy, and use of Imatinib mesylate. Mohs micrographic surgery (MMS) is the treatment of choice for DFSP as it allows for clearing of microscopic residual of the tumour and sparing of significant normal tissue not invaded by the tumour. 11,17,18 Recurrence rate following wide local excision has been well documented in the literature and ranges from 11% to 53%19–21, as an excision width of up to 10 cm may not have cleared the microscopic extent of some tumours, despite taking a huge excess of normal tissue. 11
DFSP is a radiosensitive tumour and radiation to doses of 50-60 Gray may be considered as an adjuvant to resection if margins are positive. Combined conservation resection and postoperative radiation may also be considered when adequate wide excision alone would result in major cosmetic or functional deficits. 22
Imatinib mesylate is a potent, selective inhibitor of the platelet-derived growth factor receptor (PDGFR) alpha and PDGFR beta protein-tyrosine kinase, and has been reported to induce complete or partial remission in most patients treated for advanced lesion with the t(17; 22) translocation (seen in 90% of DFSP patients). 17,23 It is used as an adjuvant in the treatment of metastatic, recurrent or unresectable disease. 23
The financial burden involved in managing malignancies is high in most settings, but are particularly overwhelming here due to poverty and lack of health insurance as only 5% of Nigerians have been enrolled into the health insurance scheme. 24 Out of pocket payment for healthcare increases financial hardship and worsens poverty index of patients and their families resulting in late presentation, inability to keep up with treatment plan, noncompliance with follow up and worsening prognosis. Effort must be made locally and nationally to incorporate everyone and especially those in the informal sector into either the national, state or community-based Health Insurance Scheme, which may be more affordable and accessible.
Conclusion
Managing dermatofibrosarcoma protuberans (DFSP) amidst delayed presentation, significant local invasion, and financial constraints presents considerable challenges. The use of chemotherapy and radiotherapy may offer improved prognosis. However, achieving a cure requires a holistic, multidisciplinary approach that includes the participation of social workers.
References
1. Leanne E.What is Dermatofibrosarcoma Protuberans? http://sarcomahelp.org/dermatofibrosarcoma-protuberans.html#tpm1_1, Accessed on 23/01/2017.
2. Lemm D, Mügge L-O, Mentzel T, Höffken K. Current treatment options in dermatofibrosarcoma protuberans. Journal of cancer research and clinical oncology. 2009;135(5):653-665. doi:10.1007/s00432-009-0550-3.
3. Darier J, Ferrand M: Dermatofibromes progressifs et recidivants ou fibrosarcomes de la peau. Ann Dermatol Syphiliga 1924; 5:545-562.
4. Gloster HM. Dermatofibrosarcoma protuberans. Journal of the American Academy of Dermatology. 1996;35(3 Pt 1):355-374; http://www.ncbi.nlm.nih.gov/pubmed/8784271.
5. Bowne WB, Antonescu CR, Leung DH, Katz SC, Hawkins WG, Woodruff JM, Brennan MF, Lewis JJ. Dermatofibrosarcoma protuberans: A clinicopathologic analysis of patients treated and followed at a single institution. Cancer. 2000;88(12):2711-2720.
6. Taylor HB, Helwig EB: Dermatofibrosarcoma protuberans: A study of 115 cases. Cancer 1962; 15:717-725.
7. Krish T, Lester F. Dermatofibrosarcoma Protuberans (DFSP), http://www.dovemed.com/dermatofibrosarcoma-protuberans-dfsp/ Accessed on 23/01/2017.
8. Minter RM, Reith JD., Metastatic potential of dermatofibrosarcoma protuberans with fibrosarcomatous change. J Surg Oncol. 2003;82(3):201-208.
9. Kreicher KL, Kurlander DE, Gittleman HR, Barnholtz-Sloan JS. Incidence and Survival of Primary Dermatofibrosarcoma Protuberans in the United States. dermatol surge. 2006;42(1):24-31.
10. Criscione VD, Weinstock MA. Descriptive epidemiology of dermatofibrosarcoma protuberans in the United States, 1973 to 2002. J Am Acad Dermatol. 2007;56(6):968-973.
11. Ratner D, Thomas CO, Johnson TM, Sondak VK, Hamilton TA, Nelson BR, Swanson NA, Garcia C, Clark RE GD. Mohs micrographic surgery for the treatment of dermatofibrosarcoma protuberans. Results of a multi-institutional series with an analysis of the extent of microscopic spread. J Am Acad Dermatol. 1997;37(4):600-613.
12. Monsudi KF, Ibrahim MH, Ayodapo AO, Owoeye JFA, Na‘allah GR. Dermatofibrosarcoma Protuberans of the Scalp and Lid - A Huge Challenge: Case Report. Nigeria: World Journal of Biomedical Research [Internet]. 2023; 10(2):66-70.
13. Effiom OA, Olojede ACO, Akinde OR, Olawuyi AB, Amoo AT, Arotiba GT. Dermatofibrosarcoma protuberans: Clinicopathologic presentation in Nigerians. Pan African Medical Journal. 2018;31:25. doi:10.11604/pamj.2018.31.25.13665.
14. Ogun GO, Ezenkwa US, Ayandipo OO. Dermatofibrosarcoma protuberance in a black African cohort - A clinicopathologic study. ecancermedicalscience. 2020;14:1086 doi:10.3332/ECANCER.2020.1086.
15. Laskin WB. Dermatofibrosarcoma protuberans. Ca-A Cancer Journal for Clinicians. 1992;42:116-125.
16. Angouridakis N, Kafas P, Jerjes W. Dermatofibrosarcoma protuberans with fibrosarcomatous transformation of the head and neck. Head and Neck Oncology. 2011;3(1):3-5.
17. Sanjay Bhambri, Avani Desai, James Rosso, Narciss Mobini. Dermatofibrosarcoma Protuberans A Case Report and Review of the Literature Sanjay Bhambri. J Clin Aesthet Dermatol. 2008;1(1):34-36.
18. Gloster HM Jr, Harris KR, Roenigk RK. A comparison between Mohs micrographic surgery and wide surgical excision for the treatment of dermatofibrosarcoma protuberans. J Am Acad Dermatol. 1996;35(1):82-87.
19. Burud IA, How NS, CheeWei G, Roslina S. Dermatofibrosarcoma Protuberance of the Breast: a Diagnostic Challenge. Indian Journal of Surgery. 2017;79(2):169-172. doi:10.1007/s12262-016-1502-1.
20. Mani S, Kumar R, Kakkar A. Recurrent Dermatofibrosarcoma Protuberans of the Head and Neck: A Case Series. Indian Journal of Surgical Oncology. 2023;14(1):128-136. doi:10.1007/s13193-022-01636-1.
21. Ali S, Ahmad I, Yaseen M, Sudhy I. Recurrent dermatofibrosarcoma protuberance: A single-center analysis. Turkish Journal of Plastic Surgery. 2023;31(4):136. doi:10.4103/tjps.tjps_30_23.
22. Ballo MT, Zagars GK, Pisters P, Pollack A. The role of radiation therapy in the management of dermatofibrosarcoma protuberans. International journal of radiation oncology, biology, physics. 1998;40(4):823-827. doi:10.1016/S0360-3016(97)00895-X.
23. Abrams TA, Schuetze SM. Targeted therapy for dermatofibrosarcoma protuberans. Current Oncology Reports. 2006;8(4):291-296. doi:10.1007/s11912-006-0035-3.
24. Alawode GO, Adewole DA. Assessment of the design and implementation challenges of the National Health Insurance Scheme in Nigeria: a qualitative study among sub-national level actors, healthcare and insurance providers. BMC Public Health. 2021;21(1):124-125. doi:10.1186/s12889-020-10133-5.
.